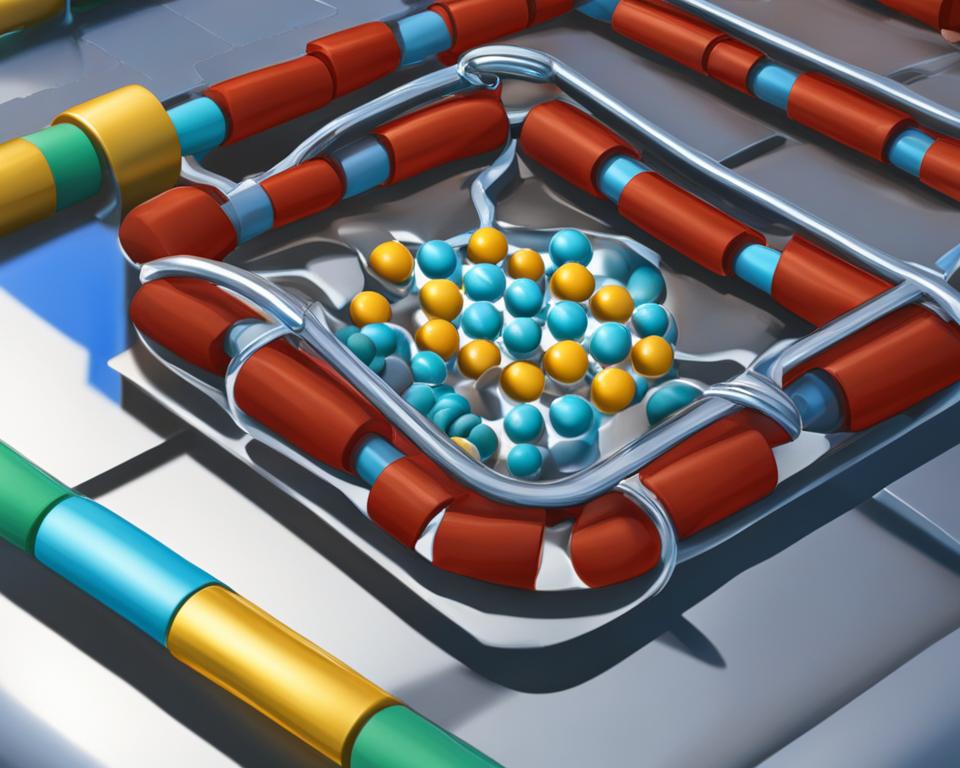
The lock and key model is a fundamental concept in understanding enzyme-substrate interaction, enzyme specificity, and enzyme catalysis. This model, first proposed by Emil Fischer in 1894, describes the specific complementary shapes of enzymes and substrates, which allow for precise binding and catalytic activity.
In the lock and key model, enzymes and substrates are likened to a lock and key, respectively. Just as a key fits perfectly into a lock, enzymes and substrates have complementary shapes that fit together tightly. This specific fit enables enzymes to catalyze chemical reactions with high specificity, meaning they can selectively bind and catalyze specific reactions.
The lock and key model emphasizes the importance of the active site in enzymes. The active site is the region where the substrate binds and catalytic reactions take place. It possesses a specific shape and chemical environment that facilitate precise binding and catalysis.
While the lock and key model is a valuable framework, it has some limitations. It does not fully explain how the enzyme-substrate complex is stabilized during the transition state of a reaction, and it assumes that enzymes are rigid structures, which may not always be the case. The induced fit model, proposed as an alternative, suggests that the active site can change its shape to better accommodate the substrate.
Key Takeaways:
- The lock and key model describes the specific complementary shapes of enzymes and substrates.
- Enzymes catalyze reactions with high specificity by selectively binding and catalyzing specific reactions.
- The active site of an enzyme is crucial for binding and catalytic activity.
- The lock and key model has limitations and the induced fit model offers an alternative perspective.
- Understanding enzyme-substrate interaction is essential for studying enzyme specificity and catalysis.
Understanding Enzyme-Substrate Interaction
Enzyme-substrate interaction lies at the heart of the lock and key model. It is the process by which enzymes and substrates bind together to form an enzyme-substrate complex. In this model, the binding between the enzyme and substrate is highly specific, with their shapes fitting together like a lock and key. This specific fit allows for the precise alignment of molecules, facilitating catalysis in living organisms.
The induced fit model, proposed by Daniel Koshland, builds upon the lock and key model by suggesting that the active site of the enzyme can slightly change its shape upon substrate binding. This flexibility enables the active site to undergo a conformational change, enhancing the precise fit between the enzyme and substrate. This refined fit optimizes catalysis and increases the efficiency of enzyme-substrate interactions.
Enzyme activation and inhibition are two important aspects of enzyme function. Enzyme activation refers to the process by which an enzyme becomes fully functional and capable of catalyzing a reaction. On the other hand, enzyme inhibition involves the reduction or blocking of enzyme activity. These regulatory mechanisms play a crucial role in maintaining the balance of enzyme activity within the cell, allowing for precise control over metabolic processes.
| Enzyme-Substrate Complex | Induced Fit Model | Enzyme Activation and Inhibition |
|---|---|---|
| The enzyme and substrate bind to form a complex | The active site of the enzyme can change shape upon substrate binding | Enzyme activation makes the enzyme functional; inhibition reduces or blocks enzyme activity |
| Highly specific binding | Precise fit enhances catalysis | Mechanisms to control enzyme activity |
The lock and key model, along with the induced fit model, provides valuable insights into the intricate nature of enzyme-substrate interactions. By understanding these concepts, scientists can decipher the mechanisms underlying enzyme specificity, catalysis, and control. These fundamental principles pave the way for advancements in fields such as medicine, bioengineering, and biotechnology, where harnessing the power of enzymes is of paramount importance.
Emil Fischer and the Lock and Key Model
One of the key figures in the development of the lock and key model was Emil Fischer, a renowned German scientist. In 1894, Fischer proposed the concept that enzymes exhibit high specificity towards their substrates, laying the foundation for our understanding of enzyme-substrate interaction. Fischer’s work focused on the idea that enzymes and substrates possess specific complementary shapes and sizes, allowing for precise binding and catalysis.
The lock and key model, as postulated by Fischer, explains how enzymes act as the “locks” and substrates as the “keys” in a molecular interaction. Enzyme specificity refers to the ability of enzymes to selectively bind and catalyze specific reactions with their substrates. Fischer’s work helped elucidate the unique ability of enzymes to choose the correct substrate from a group of similar molecules.
“Enzymes are substances whose molecules possess a peculiar property, known as catalytic power, enabling them to bring about chemical transformations of matter.”
Fischer’s lock and key model was a significant breakthrough in our understanding of enzyme specificity and substrate specificity. It provided a framework to explain the precise binding between enzymes and their specific substrates, leading to high selectivity and catalytic efficiency. This model revolutionized the field of enzymology and laid the groundwork for further investigations into the fascinating world of enzymes and their vital role in biological processes.
Table: Comparing the Lock and Key Model and the Induced Fit Model
| Feature | Lock and Key Model | Induced Fit Model |
|---|---|---|
| Molecular Interaction | Enzyme and substrate have specific complementary shapes for precise binding | Enzyme’s active site changes shape upon substrate binding for a more precise fit |
| Enzyme Flexibility | Assumes enzymes are rigid structures | Recognizes that enzymes can undergo conformational changes |
| Transition State Stabilization | Does not fully explain how enzyme-substrate complex is stabilized during the transition state of a reaction | Suggests enzyme undergoes conformational changes to optimize substrate binding during the transition state |
This table compares the key features of the lock and key model and the induced fit model, proposed as an alternative to the lock and key model. While Fischer’s lock and key model provided a fundamental understanding of enzyme-substrate interaction, the induced fit model offers a more refined perspective, considering enzyme flexibility and the transition state stabilization. Both models contribute to our comprehension of the complex world of enzymes and their specificity towards substrates.
Active Sites and Enzyme Function
The active site of an enzyme is a critical component in its function. It acts as a specialized region where the enzyme and substrate come together to facilitate catalysis. The active site has a unique shape and chemical environment that allows for the specific binding of the substrate.
Enzyme catalysis occurs when the enzyme and substrate interact at the active site, resulting in the conversion of the substrate into a product. This process involves the formation of temporary bonds and the breaking of existing bonds within the substrate molecule. The active site provides the necessary conditions for these reactions to take place efficiently.
The lock and key model highlights the importance of the specific complementary shape between the active site and the substrate. This precise fit allows for optimal binding, ensuring that only the appropriate substrate can interact with the enzyme. By promoting the formation of the enzyme-substrate complex, the active site plays a crucial role in facilitating catalysis.
| Enzyme Catalysis | Active Site | Enzyme-Substrate Binding |
|---|---|---|
| Process of speeding up chemical reactions | Specialized region where enzyme and substrate interact | Specific complementary shape between active site and substrate |
| Formation of temporary bonds and breaking of existing bonds | Unique shape and chemical environment | Optimal binding for the appropriate substrate |
| Facilitated by the active site for efficient catalysis | Ensures necessary conditions for reactions | Promotes formation of enzyme-substrate complex |
“The active site is like a keyhole, and the substrate is the corresponding key. Only when the key fits perfectly into the keyhole can the door be unlocked and the reaction proceed.”
In summary, the active site of an enzyme is a specialized region that plays a crucial role in enzyme function. It provides the necessary conditions for efficient catalysis and promotes the specific binding of the substrate. The lock and key model helps to explain the importance of the active site’s complementary shape in facilitating enzyme-substrate interactions and ensuring the specificity of enzymatic reactions. Understanding the role of active sites enhances our understanding of enzyme catalysis and its significance in biological processes.
Enzyme Specificity and Substrate Specificity
Enzyme specificity and substrate specificity are fundamental concepts in understanding the lock and key model of enzyme-substrate interaction. Enzymes display a remarkable ability to selectively bind and catalyze specific reactions, contributing to the efficiency and specificity of biochemical processes in living organisms.
The lock and key model, introduced by Emil Fischer in 1894, elucidates how enzymes and substrates possess complementary shapes and sizes, akin to a lock and key mechanism. This specific fit allows enzymes to recognize and bind their specific substrates, resulting in selective catalysis. Enzyme specificity refers to an enzyme’s overall ability to catalyze specific reactions, whereas substrate specificity pertains to an enzyme’s capacity to recognize and bind to a particular substrate.
“Enzyme specificity ensures that the right enzyme can precisely recognize and catalyze the right substrate, contributing to the high selectivity of enzymatic reactions.”
To further illustrate the concept of enzyme specificity and substrate specificity, consider the following examples:
- An enzyme called lactase exhibits high specificity for lactose, the sugar found in milk. Lactase binds to lactose specifically and catalyzes its breakdown into simpler sugars.
- The enzyme amylase is specific to starch and breaks down the complex carbohydrate into smaller sugar molecules.
In these instances, the enzymes demonstrate their selectivity by specifically recognizing and binding to their respective substrates, resulting in highly efficient and specific catalysis.
| Enzyme | Substrate |
|---|---|
| Lactase | Lactose |
| Amylase | Starch |
Enzyme-Substrate Selectivity
Enzyme-substrate selectivity refers to the ability of enzymes to specifically choose and bind to their respective substrates, excluding others. This selectivity is a result of the complementary shapes and chemical interactions between the enzyme’s active site and the substrate molecules.
Enzyme-substrate selectivity plays a crucial role in maintaining the specificity and functionality of biochemical reactions. It ensures that enzymes interact only with their intended substrates, preventing unwanted reactions and promoting efficiency in cellular processes.
“Enzyme-substrate selectivity guarantees that enzymes make the right connections, enabling precise catalytic reactions with high efficiency.”
Overall, the lock and key model provides a valuable framework for understanding enzyme specificity and substrate specificity. By elucidating the specific complementary shapes and sizes of enzymes and substrates, this model helps explain the tight and selective interactions that lead to efficient catalysis in living organisms.
Limitations of the Lock and Key Model
The lock and key model, while widely accepted, has its limitations when it comes to fully explaining enzyme-substrate interaction. One of the key limitations is that it fails to provide a complete understanding of how the enzyme-substrate complex is stabilized during the transition state of a reaction. This transitional state is a crucial stage where chemical bonds are broken and formed, and the lock and key model does not account for the dynamic changes that occur in the active site during this process.
Another limitation of the lock and key model is its assumption that enzymes are rigid structures. In reality, enzymes can exhibit conformational changes upon substrate binding. This led to the proposal of an alternative model known as the induced fit model. The induced fit model suggests that the active site of the enzyme can change its shape slightly upon substrate binding, allowing for a more precise fit and enhanced catalysis.
The induced fit model offers a more refined view of enzyme action by incorporating the dynamic nature of enzyme-substrate interactions. It recognizes that enzymes are not static structures but can exhibit flexibility to accommodate the substrate. This model provides a better explanation for the observed variations in enzyme specificity and catalysis.
Table: Comparison of the Lock and Key Model and the Induced Fit Model
| Aspect | Lock and Key Model | Induced Fit Model |
|---|---|---|
| Enzyme-Substrate Binding | Highly specific, complementary shapes | Flexible binding, shape changes in the active site |
| Enzyme Activation | Assumes enzymes are already fully functional | Allows for conformational changes upon activation |
| Enzyme Inhibition | Does not directly address inhibition mechanisms | Can explain competitive and non-competitive inhibition |
The induced fit model builds upon the concepts of the lock and key model and provides a more comprehensive understanding of enzyme-substrate interaction. It highlights the dynamic nature of enzymes and the importance of conformational changes in substrate binding and catalysis.
Conclusion
The lock and key model plays a vital role in understanding enzyme-substrate interaction and enzyme specificity. This widely accepted model explains how enzymes and substrates possess specific complementary shapes, allowing for precise binding and catalysis.
Although the lock and key model has its limitations, it serves as a foundation for exploring enzyme-substrate interactions further. By recognizing the importance of enzyme-substrate interaction, we gain insight into the fascinating world of enzyme catalysis within living organisms.
Understanding the lock and key model provides us with valuable knowledge about the intricate mechanisms underlying enzyme specificity. This insight helps us appreciate the crucial role enzymes play in regulating chemical reactions and maintaining the delicate balance of biological processes.
FAQ
What is the lock and key model?
The lock and key model is a widely accepted model for enzyme-substrate interaction. It proposes that enzymes and substrates have specific complementary shapes that fit together like a key into a lock, allowing for precise binding and catalysis.
How does the lock and key model explain enzyme specificity?
The lock and key model suggests that enzymes exhibit high specificity towards their substrates because they have specific complementary shapes and sizes. This allows enzymes to selectively bind to and catalyze specific reactions.
What is the active site of an enzyme?
The active site is the region of an enzyme where the substrate binds and catalysis occurs. It has a specific shape and chemical environment that is essential for enzyme-substrate binding and catalysis.
What is enzyme catalysis?
Enzyme catalysis refers to the process by which enzymes speed up chemical reactions. This is facilitated by the active site of the enzyme, which allows for the precise binding and catalysis of the substrate.
What are enzyme specificity and substrate specificity?
Enzyme specificity refers to an enzyme’s overall ability to catalyze specific reactions, while substrate specificity refers to its ability to recognize and bind to a particular substrate. The lock and key model helps explain the tight interaction between enzymes and their specific substrates, leading to high selectivity.
What are the limitations of the lock and key model?
One limitation is that it does not fully explain how the enzyme-substrate complex is stabilized during the transition state of a reaction. Additionally, the model assumes that enzymes are rigid structures, which is not always the case. The induced fit model provides a more refined view of enzyme action.
![Ray Dalio Quotes [Principles, Life, Investment]](https://tagvault.org/wp-content/uploads/2023/04/Screen-Shot-2023-04-19-at-7.57.49-PM.png)