Here’s a comprehensive list of all the elements in the periodic table in order of their atomic number.
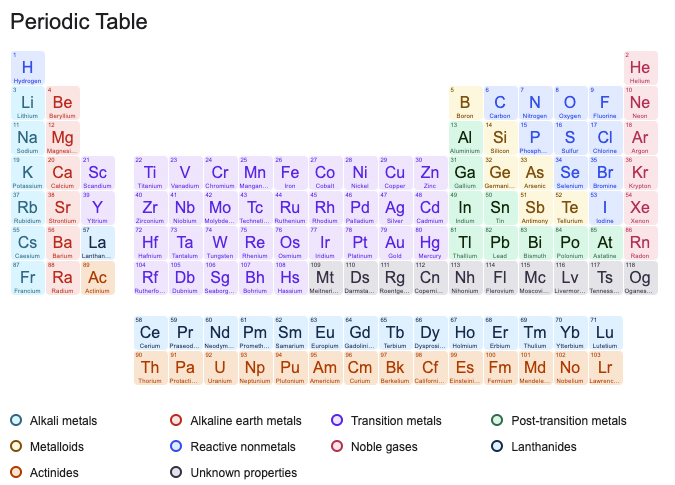
We’ll include the element’s name, symbol, atomic number, atomic weight, and a brief description of the element:
Periodic Table with Names & Symbols, Atomic Number, Atomic Weight, and Descriptions
- Hydrogen (H), atomic number 1, atomic weight 1.008, a colorless, odorless gas, the most abundant element in the universe and the first element on the periodic table.
- Helium (He), atomic number 2, atomic weight 4.0026, a colorless, odorless gas, the second most abundant element in the universe, and is named after the Greek god of the sun, Helios.
- Lithium (Li), atomic number 3, atomic weight 6.94, a soft, silvery-white metal, the lightest metal, and is used in rechargeable batteries.
- Beryllium (Be), atomic number 4, atomic weight 9.0122, a hard, gray-white metal, used in nuclear reactors and aerospace technology.
- Boron (B), atomic number 5, atomic weight 10.81, a hard, black or brown metalloid, used in heat-resistant materials, and is an essential nutrient for plants.
- Carbon (C), atomic number 6, atomic weight 12.011, a nonmetal, the basis of all known life on Earth, and is used in a variety of applications, including fuel, graphite, and diamond.
- Nitrogen (N), atomic number 7, atomic weight 14.007, a nonmetal, makes up about 78% of the Earth’s atmosphere, and is used in fertilizers, explosives, and other industrial applications.
- Oxygen (O), atomic number 8, atomic weight 15.999, a nonmetal, makes up about 21% of the Earth’s atmosphere, and is essential for respiration and combustion.
- Fluorine (F), atomic number 9, atomic weight 18.998, a highly reactive halogen gas, used in toothpaste, refrigerants, and other industrial applications.
- Neon (Ne), atomic number 10, atomic weight 20.180, a colorless, odorless gas, used in lighting and advertising signs.
- Sodium (Na), atomic number 11, atomic weight 22.990, a soft, silvery-white metal, reacts violently with water, and is used in the production of chemicals and as a flavor enhancer.
- Magnesium (Mg), atomic number 12, atomic weight 24.305, a shiny, gray-white metal, used in lightweight alloys, as a supplement, and in medical applications.
- Aluminum (Al), atomic number 13, atomic weight 26.982, a lightweight, silver-gray metal, used in construction, packaging, and transportation.
- Silicon (Si), atomic number 14, atomic weight 28.086, a metalloid, the second most abundant element in the Earth’s crust, used in electronics, solar cells, and other industrial applications.
- Phosphorus (P), atomic number 15, atomic weight 30.974, a nonmetal, an essential nutrient for plants and animals, and is used in fertilizers, matches, and other industrial applications.
- Sulfur (S), atomic number 16, atomic weight 32.06, a nonmetal, used in fertilizers, detergents, and other industrial applications.
- Chlorine (Cl), atomic number 17, atomic weight 35.45, a highly reactive halogen gas, used in water treatment, bleach, and other industrial applications.
- Argon (Ar), atomic number 18, atomic weight 39.948, a colorless, odorless gas, the third most abundant gas in the Earth’s atmosphere, used in welding, lighting, and other industrial applications.
- Potassium (K), atomic number 19, atomic weight 39.098, a soft, silvery-white metal, highly reactive with water, and is an essential nutrient for plants and animals.
- Calcium (Ca), atomic number 20, atomic weight 40.078, a silvery-white metal, used in construction, medicine, and dietary supplements.
- Scandium (Sc), atomic number 21, atomic weight 44.956, a silvery-white metal, used in high-strength aluminum alloys and other industrial applications.
- Titanium (Ti), atomic number 22, atomic weight 47.867, a silver-gray metal, used in aerospace technology, medical implants, and other industrial applications.
- Vanadium (V), atomic number 23, atomic weight 50.942, a silvery-gray metal, used in steel alloys, batteries, and other industrial applications.
- Chromium (Cr), atomic number 24, atomic weight 52.00, a silvery-gray metal, used in stainless steel, plating, and other industrial applications.
- Manganese (Mn), atomic number 25, atomic weight 54.938, a silvery-gray metal, used in steel alloys, batteries, and other industrial applications.
- Iron (Fe), atomic number 26, atomic weight 55.845, a silvery-gray metal, the most common element on Earth by mass, used in construction, tools, and other industrial applications.
- Cobalt (Co), atomic number 27, atomic weight 58.933, a silvery-gray metal, used in magnets, batteries, and other industrial applications.
- Nickel (Ni), atomic number 28, atomic weight 58.693, a silvery-white metal, used in coins, batteries, and other industrial applications.
- Copper (Cu), atomic number 29, atomic weight 63.546, a reddish-orange metal, used in electrical wiring, plumbing, and other industrial applications.
- Zinc (Zn), atomic number 30, atomic weight 65.38, a bluish-white metal, used in galvanizing, batteries, and other industrial applications.
- Gallium (Ga), atomic number 31, atomic weight 69.723, a silvery-white metal, used in semiconductors and other industrial applications.
- Germanium (Ge), atomic number 32, atomic weight 72.630, a gray-white metalloid, used in semiconductors, fiber optic cables, and other industrial applications.
- Arsenic (As), atomic number 33, atomic weight 74.922, a gray metalloid, used in pesticides, wood preservatives, and other industrial applications.
- Selenium (Se), atomic number 34, atomic weight 78.960, a nonmetal, used in photovoltaic cells, glass production, and other industrial applications.
- Bromine (Br), atomic number 35, atomic weight 79.904, a highly reactive halogen liquid, used in flame retardants, water treatment, and other industrial applications.
- Krypton (Kr), atomic number 36, atomic weight 83.798, a colorless, odorless gas, used in lighting and other industrial applications.
- Rubidium (Rb), atomic number 37, atomic weight 85.468, a soft, silvery-white metal, highly reactive with water, and is used in atomic clocks and other industrial applications.
- Strontium (Sr), atomic number 38, atomic weight 87.62, a silvery-white metal, used in flares, fireworks, and other pyrotechnic applications, as well as in medicine for bone density imaging.
- Yttrium (Y), atomic number 39, atomic weight 88.906, a silvery-metallic transition metal, used in phosphors for color television tubes and other electronic applications, as well as in superconductors and other industrial applications.
- Zirconium (Zr), atomic number 40, atomic weight 91.224, a silvery-white metal, used in nuclear reactors, as well as in high-performance alloys for jet engines, gas turbines, and other industrial applications.
- Niobium (Nb), atomic number 41, atomic weight 92.906, a soft, gray, ductile metal, used in superconductors, jet engines, and other high-tech applications.
- Molybdenum (Mo), atomic number 42, atomic weight 95.94, a silvery-white metal, used in high-strength alloys, electrical contacts, and other industrial applications.
- Technetium (Tc), atomic number 43, atomic weight 98, an artificially produced radioactive element, used in medical imaging.
- Ruthenium (Ru), atomic number 44, atomic weight 101.07, a hard, white metal, used in alloys with platinum and palladium, as well as in electronic applications.
- Rhodium (Rh), atomic number 45, atomic weight 102.91, a silvery-white metal, used in catalytic converters, as well as in jewelry and other decorative applications.
- Palladium (Pd), atomic number 46, atomic weight 106.42, a silvery-white metal, used in catalytic converters, electronics, and jewelry.
- Silver (Ag), atomic number 47, atomic weight 107.87, a soft, white, lustrous transition metal, used in jewelry, currency, and other decorative applications, as well as in electronics and other industrial applications.
- Cadmium (Cd), atomic number 48, atomic weight 112.41, a soft, bluish-white metal, used in batteries, pigments, and other industrial applications, but also considered toxic and potentially carcinogenic.
- Indium (In), atomic number 49, atomic weight 114.82, a soft, silvery-white metal, used in electronics, solar panels, and other industrial applications.
- Tin (Sn), atomic number 50, atomic weight 118.71, a silvery-white metal, used in tin plating, solders, and other industrial applications.
- Antimony (Sb), atomic number 51, atomic weight 121.76, a lustrous gray metalloid, used in flame retardants, batteries, and other industrial applications.
- Tellurium (Te), atomic number 52, atomic weight 127.60, a brittle, silver-white metalloid, used in semiconductors, solar cells, and other industrial applications.
- Iodine (I), atomic number 53, atomic weight 126.90, a dark grayish-purple nonmetal, used in disinfectants, photography, and other industrial applications.
- Xenon (Xe), atomic number 54, atomic weight 131.29, a colorless, odorless gas, used in lighting, medical imaging, and other industrial applications.
- Cesium (Cs), atomic number 55, atomic weight 132.91, a soft, silvery-gold alkali metal, used in atomic clocks and other precision timekeeping devices, as well as in research applications.
- Barium (Ba), atomic number 56, atomic weight 137.33, a soft, silvery-white alkaline earth metal, used in barium meals for medical imaging, as well as in drilling muds, fireworks, and other industrial applications.
- Lanthanum (La), atomic number 57, atomic weight 138.91, a silvery-white metallic rare earth element, used in carbon arc lighting, as well as in batteries, catalytic converters, and other industrial applications.
- Cerium (Ce), atomic number 58, atomic weight 140.12, a silvery-white metallic rare earth element, used in catalytic converters, as well as in polishing compounds, glass manufacturing, and other industrial applications.
- Praseodymium (Pr), atomic number 59, atomic weight 140.91, a silvery-white metallic rare earth element, used in magnets, lasers, and other high-tech applications.
- Neodymium (Nd), atomic number 60, atomic weight 144.24, a silvery-white metallic rare earth element, used in magnets, lasers, and other high-tech applications.
- Promethium (Pm), atomic number 61, atomic weight 145, an artificially produced radioactive rare earth element, used in research and medical imaging.
- Samarium (Sm), atomic number 62, atomic weight 150.36, a silvery-white metallic rare earth element, used in magnets, lasers, and other high-tech applications.
- Europium (Eu), atomic number 63, atomic weight 151.96, a silvery-white metallic rare earth element, used in phosphors for television screens and other electronic displays.
- Gadolinium (Gd), atomic number 64, atomic weight 157.25, a silvery-white metallic rare earth element, used in magnetic resonance imaging (MRI), as well as in neutron capture therapy for cancer.
- Terbium (Tb), atomic number 65, atomic weight 158.93, a silvery-white metallic rare earth element, used in phosphors for lighting and other electronic applications.
- Dysprosium (Dy), atomic number 66, atomic weight 162.50, a silvery-white metallic rare earth element, used in magnets, lasers, and other high-tech applications.
- Holmium (Ho), atomic number 67, atomic weight 164.93, a silvery-white metallic rare earth element, used in nuclear control rods, as well as in magnets and other high-tech applications.
- Erbium (Er), atomic number 68, atomic weight 167.26, a silvery-white metallic rare earth element, used in fiber-optic communication systems, as well as in nuclear control rods and other industrial applications.
- Thulium (Tm), atomic number 69, atomic weight 168.93, a silvery-white metallic rare earth element, used in portable x-ray machines and other medical and research applications.
- Ytterbium (Yb), atomic number 70, atomic weight 173.05, a silvery-white metallic rare earth element, used in atomic clocks, lasers, and other high-tech applications.
- Lutetium (Lu), atomic number 71, atomic weight 174.97, a silvery-white metallic rare earth element, used in radiation therapy for cancer, as well as in other medical and research applications.
- Hafnium (Hf), atomic number 72, atomic weight 178.49, a lustrous, silvery-white metal, used in nuclear reactors, as well as in alloys for high-temperature applications.
- Tantalum (Ta), atomic number 73, atomic weight 180.95, a hard, dense, blue-gray metal, used in electronic capacitors, as well as in surgical implants and other medical applications.
- Tungsten (W), atomic number 74, atomic weight 183.84, a dense, steel-gray metal, used in filaments for incandescent light bulbs, as well as in high-strength alloys for aircraft and other applications.
- Rhenium (Re), atomic number 75, atomic weight 186.21, a silvery-white metal with a high melting point, used in high-temperature alloys, as well as in filaments for mass spectrometers and other scientific instruments.
- Osmium (Os), atomic number 76, atomic weight 190.23, a dense, bluish-white metal, used in alloys for electrical contacts, fountain pen tips, and other applications.
- Iridium (Ir), atomic number 77, atomic weight 192.22, a dense, silvery-white metal, used in alloys for electrical contacts, spark plugs, and other applications, as well as in the production of high-strength alloys for aircraft and other applications.
- Platinum (Pt), atomic number 78, atomic weight 195.08, a dense, silvery-white metal, used in jewelry, as well as in catalytic converters, electrical contacts, and other industrial applications.
- Gold (Au), atomic number 79, atomic weight 196.97, a soft, yellow, dense metal, used in jewelry, as well as in electrical contacts, dental fillings, and other industrial applications.
- Mercury (Hg), atomic number 80, atomic weight 200.59, a silvery-white metal that is liquid at room temperature, used in thermometers, fluorescent lamps, and other applications, as well as in some industrial processes.
- Thallium (Tl), atomic number 81, atomic weight 204.38, a soft, bluish-gray metal, used in electronic switches, as well as in rat poisons and other toxic compounds.
- Lead (Pb), atomic number 82, atomic weight 207.2, a dense, bluish-gray metal, used in batteries, as well as in plumbing, soldering, and other industrial applications.
- Bismuth (Bi), atomic number 83, atomic weight 208.98, a silvery-white metal with a pinkish tint, used in cosmetics, as well as in pharmaceuticals, alloys, and other industrial applications.
- Polonium (Po), atomic number 84, atomic weight 209, a highly radioactive, silvery-gray metal, used in research and some industrial applications.
- Astatine (At), atomic number 85, atomic weight 210, a highly radioactive, rare, and unstable halogen element, used in research and some medical applications.
- Radon (Rn), atomic number 86, atomic weight 222, a highly radioactive, colorless, and odorless gas, used in some industrial and scientific applications, as well as in cancer therapy.
- Francium (Fr), atomic number 87, atomic weight 223, a highly radioactive alkali metal, used only in research.
- Radium (Ra), atomic number 88, atomic weight 226, a highly radioactive alkaline earth metal, used in some medical and industrial applications.
- Actinium (Ac), atomic number 89, atomic weight 227, a highly radioactive metal, used only in research.
- Thorium (Th), atomic number 90, atomic weight 232.04, a naturally occurring radioactive metal, used in nuclear reactors, as well as in some high-temperature alloys.
- Protactinium (Pa), atomic number 91, atomic weight 231.04, a highly radioactive metal, used only in research.
- Uranium (U), atomic number 92, atomic weight 238.03, a naturally occurring radioactive metal, used in nuclear reactors and weapons, as well as in some medical and industrial applications.
- Neptunium (Np), atomic number 93, atomic weight 237.05, a highly radioactive metal, used only in research.
- Plutonium (Pu), atomic number 94, atomic weight 244, a highly radioactive metal, used in nuclear weapons and reactors, as well as in some space exploration applications.
- Americium (Am), atomic number 95, atomic weight 243, a highly radioactive metal, used in smoke detectors, as well as in some medical and industrial applications.
- Curium (Cm), atomic number 96, atomic weight 247, a highly radioactive metal, used only in research.
- Berkelium (Bk), atomic number 97, atomic weight 247, a highly radioactive metal, used only in research.
- Californium (Cf), atomic number 98, atomic weight 251, a highly radioactive metal, used only in research.
- Einsteinium (Es), atomic number 99, atomic weight 252, a highly radioactive metal, used only in research.
- Fermium (Fm), atomic number 100, atomic weight 257, a highly radioactive metal, used only in research.
- Mendelevium (Md), atomic number 101, atomic weight 258, a highly radioactive metal, used only in research.
- Nobelium (No), atomic number 102, atomic weight 259, a highly radioactive metal, used only in research.
- Lawrencium (Lr), atomic number 103, atomic weight 262, a highly radioactive metal, used only in research.
- Rutherfordium (Rf), atomic number 104, atomic weight 267, a synthetic radioactive metal, used only in research.
- Dubnium (Db), atomic number 105, atomic weight 270, a synthetic radioactive metal, used only in research.
- Seaborgium (Sg), atomic number 106, atomic weight 271, a synthetic radioactive metal, used only in research.
- Bohrium (Bh), atomic number 107, atomic weight 270, a synthetic radioactive metal, used only in research.
- Hassium (Hs), atomic number 108, atomic weight 277, a synthetic radioactive metal, used only in research.
- Meitnerium (Mt), atomic number 109, atomic weight 278, a synthetic radioactive metal, used only in research.
- Darmstadtium (Ds), atomic number 110, atomic weight 281, a synthetic radioactive metal, used only in research.
- Roentgenium (Rg), atomic number 111, atomic weight 282, a synthetic radioactive metal, used only in research.
- Copernicium (Cn), atomic number 112, atomic weight 285, a synthetic radioactive metal, used only in research.
- Nihonium (Nh), atomic number 113, atomic weight 284, a synthetic radioactive metal, used only in research.
- Flerovium (Fl), atomic number 114, atomic weight 289, a synthetic radioactive metal, used only in research.
- Moscovium (Mc), atomic number 115, atomic weight 288, a synthetic radioactive metal, used only in research.
- Livermorium (Lv), atomic number 116, atomic weight 293, a synthetic radioactive metal, used only in research.
- Tennessine (Ts), atomic number 117, atomic weight 294, a synthetic radioactive metal, used only in research.
- Oganesson (Og), atomic number 118, atomic weight 294, a synthetic radioactive metal, used only in research.
Note that some of the elements beyond atomic number 92 are synthetic, meaning they are not naturally occurring and have only been produced in the laboratory.
They are typically highly unstable and radioactive, with very short half-lives, which makes them challenging to study and use in applications beyond basic scientific research.