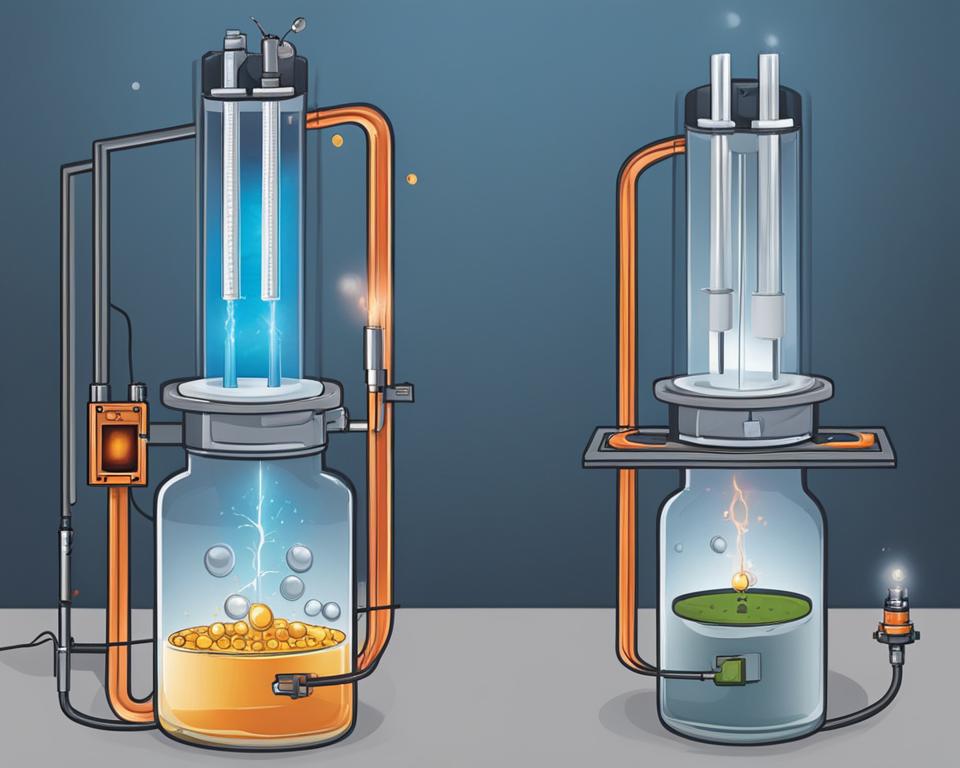
Welcome to our article on voltaic vs electrolytic cells! If you’ve ever wondered about the differences and similarities between these two types of electrochemical cells, you’ve come to the right place.
In this article, we will explore the key characteristics of voltaic and electrolytic cells, their operation, and the source of energy that drives their reactions.
Key Takeaways:
- Voltaic cells generate electrical energy from spontaneous redox reactions, while electrolytic cells use electrical energy to drive non-spontaneous reactions.
- Voltaic cells produce direct current (DC) electricity and can be used as a source of electrical energy in batteries.
- Electrolytic cells are used in processes like electrolysis and electroplating.
- Voltaic cells rely on spontaneous oxidation and reduction reactions, while electrolytic cells require an external electric current to facilitate redox reactions.
- Both types of cells have electrodes and electrolytes, but their purpose and energy source differ.
Overview and Key Difference
When it comes to electrochemistry, there are two main types of electrochemical cells: voltaic cells and electrolytic cells.
These cells have significant differences in terms of their energy source and operation.
Understanding these differences is crucial in order to grasp their respective roles and applications. Let’s take a closer look at the key differences between voltaic and electrolytic cells.
Voltaic cells, also known as galvanic cells, generate electricity through spontaneous redox reactions. These reactions release energy, which is harnessed to produce an electric current.
On the other hand, electrolytic cells require an external electrical energy source to drive non-spontaneous redox reactions. In essence, voltaic cells function as energy generators, while electrolytic cells function as energy consumers.
The direction of electron flow is another distinguishing factor between voltaic and electrolytic cells.
In a voltaic cell, electrons flow from the anode to the cathode, while in an electrolytic cell, electrons flow from the cathode to the anode.
This reverse electron flow in electrolytic cells allows for non-spontaneous reactions to occur.
Table: Voltaic vs Electrolytic Cell
| Aspect | Voltaic Cell | Electrolytic Cell |
|---|---|---|
| Energy Source | Spontaneous redox reactions | External electrical energy source |
| Direction of Electron Flow | Anode to cathode | Cathode to anode |
| Purpose | Energy generation | Energy consumption |
| Operation | Release of energy | Input of energy |
By understanding the fundamental differences between voltaic and electrolytic cells, we can appreciate their unique applications and contributions in various fields.
Whether it’s harnessing energy from spontaneous reactions or driving non-spontaneous reactions, both types of cells play a crucial role in the realm of electrochemistry.
Voltaic Cell vs Electrolytic Cell in Tabular Form
When comparing voltaic cells and electrolytic cells, it is helpful to consider their key characteristics side by side. The following table provides a clear overview of the differences between these two types of electrochemical cells:
| Characteristic | Voltaic Cell | Electrolytic Cell |
|---|---|---|
| Source of Energy | Spontaneous redox reactions | Electrical energy from an external source |
| Operation | Produces electricity | Drives non-spontaneous reactions |
| Purpose | Generate electrical energy | Facilitate chemical reactions |
| Electron Flow | Anode (-) to cathode (+) | Cathode (-) to anode (+) |
As depicted in the table, voltaic cells generate electricity through spontaneous redox reactions, while electrolytic cells require an external source of electrical energy to drive non-spontaneous reactions.
Voltaic cells are commonly used as a source of electrical energy in batteries, while electrolytic cells are utilized in processes like electrolysis and electroplating.
Voltaic Cells
Voltaic cells, also known as galvanic cells, are characterized by their ability to use spontaneous redox reactions to generate electrical energy.
In these cells, the anode is the site of oxidation, where electrons are released, while the cathode is the site of reduction, where electrons are gained.
Voltaic cells produce direct current (DC) electricity and rely on a salt bridge to maintain the movement of ions between the half-cells.
Electrolytic Cells
Electrolytic cells, on the other hand, utilize electrical energy from an external source to drive non-spontaneous redox reactions. The cathode of an electrolytic cell is the site of oxidation, while the anode is the site of reduction.
These cells often involve the use of an electrolyte solution to facilitate the movement of ions between the electrodes. They find applications in processes like electrolysis and electroplating.
Voltaic Cell Characteristics
Voltaic cells, also known as galvanic cells, possess several distinct characteristics that make them unique in their ability to generate electrical energy. These characteristics include:
- Spontaneous Redox Reactions: Voltaic cells utilize spontaneous redox reactions to generate electricity. These reactions occur naturally and release energy, which is harnessed to produce an electric current.
- Direct Current (DC) Electricity: The electricity produced by voltaic cells is in the form of direct current (DC). This means that the flow of electrons is unidirectional, traveling from the anode to the cathode.
- Oxidation at the Anode: In a voltaic cell, the anode is the site of oxidation. During this process, electrons are released by the anode, contributing to the flow of electricity.
- Reduction at the Cathode: The cathode of a voltaic cell is the site of reduction. Electrons from the external circuit are gained by the cathode, completing the redox reaction.
- Source of Electrical Energy: Voltaic cells serve as a source of electrical energy and are commonly used in batteries to power various devices. As the spontaneous redox reaction takes place, electrical energy is generated and can be utilized.
- Salt Bridge: To facilitate the movement of ions between the two half-cells of a voltaic cell, a salt bridge is often used. This maintains the electrical neutrality of the solution and allows for the continued flow of electrons.
Table: Comparison of Voltaic and Electrolytic Cells
| Characteristic | Voltaic Cells | Electrolytic Cells |
|---|---|---|
| Source of Energy | Spontaneous redox reactions | External electrical energy |
| Flow of Electrons | Anode to cathode | Cathode to anode |
| Purpose | Generate electrical energy | Drive non-spontaneous reactions |
| Anode | Site of oxidation | Site of reduction |
| Cathode | Site of reduction | Site of oxidation |
| Application | Batteries | Electrolysis, electroplating |
| Electrolyte | No specific requirement | Required for ion movement |
Voltaic cells are characterized by their ability to generate electrical energy through spontaneous redox reactions.
They produce direct current (DC) electricity, with the anode serving as the site of oxidation and the cathode as the site of reduction.
These cells, also known as galvanic cells, can be used as a source of electrical energy in batteries and rely on the movement of ions facilitated by a salt bridge.
In contrast, electrolytic cells use electrical energy from an external source to drive non-spontaneous redox reactions.
The flow of electrons in electrolytic cells is typically from the cathode to the anode, with the cathode serving as the site of oxidation and the anode as the site of reduction.
These cells find applications in processes like electrolysis and electroplating. They require an electrolyte solution to enable the movement of ions between the electrodes.
Understanding the characteristics of voltaic and electrolytic cells is crucial in comprehending their respective roles in electrochemistry.
While voltaic cells generate electricity through spontaneous reactions, electrolytic cells utilize electrical energy to drive non-spontaneous reactions, highlighting the key differences between these two types of electrochemical cells.
Electrolytic Cell Characteristics
Now let’s delve into the unique characteristics of electrolytic cells. These fascinating devices operate differently from voltaic cells and serve a variety of purposes.
In contrast to voltaic cells, electrolytic cells rely on electrical energy from an external source to drive non-spontaneous redox reactions. This means that they can initiate reactions that would not occur naturally.
As a result, electrolytic cells are commonly used in processes such as electrolysis, where compounds are decomposed through the application of electric current.
Electrolytic cells facilitate the flow of electrons from the cathode to the anode. Like voltaic cells, the cathode is the site of oxidation, where electrons are released, while the anode is the site of reduction, where electrons are gained.
These cells also require an electrolyte solution to enable the movement of ions between the electrodes, ensuring the continuity of the chemical reactions taking place.
Additionally, electrolytic cells find application in processes like electroplating metals onto surfaces.
By utilizing the controlled flow of electric current, various metals can be coated onto desired materials, enhancing their appearance and functionality.
FAQ
What is the difference between a voltaic cell and an electrolytic cell?
A voltaic cell generates electrical energy from spontaneous redox reactions, while an electrolytic cell uses electrical energy to drive non-spontaneous redox reactions.
How do voltaic cells generate electricity?
Voltaic cells produce electricity through the release of energy during spontaneous reactions.
How do electrolytic cells work?
Electrolytic cells use electrical energy from an external source to drive non-spontaneous redox reactions.
What is the purpose of a voltaic cell?
Voltaic cells can be used as a source of electrical energy in batteries.
What are some applications of electrolytic cells?
Electrolytic cells are used in processes like electrolysis, which involves the decomposition of compounds through the application of electric current. They are also used for electroplating metals onto surfaces.
How do electrons flow in voltaic and electrolytic cells?
In voltaic cells, electrons flow from the anode (site of oxidation) to the cathode (site of reduction). In electrolytic cells, electrons typically flow from the cathode to the anode.